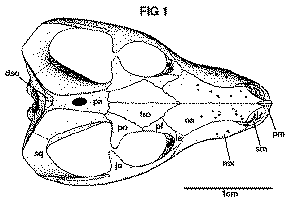
 [Fig. 1]
[Fig. 1]
digital version of this article downloaded from: T. Rowe, W. Carlson, and W. Bottorff, 1993. THRINAXODON: DIGITAL ATLAS OF THE SKULL. The University of Texas Press, CD-ROM.
Originally published in: Bulletin, Museum of Comparative Zoology, Harvard University, 1961,125: 165-180. Reprinted by permission of Museum of Comparative Zoology, Harvard University.
By Richard Estes
The material discussed here was collected by the 1947 University of California African Expedition, Southern Section, at Harrismith, Orange Free State. The locality data is as follows: from Harrismith, six miles on the Bezuitenhout Pass road, then two miles east to a farm, and one mile north to old stone corrals on the point of a hill overlooking the river. These skulls were picked up as nodules, which occur on the west and north slopes of this point. The collectors were Dr. and Mrs. Charles L. Camp. The locality is in the early Triassic Lystrosaurus zone, and is designated University of California Vertebrate Locality number V-36115.
The specimens dealt with below include two juvenile skulls, which bear University of California nos. 42877 and 42878, and two adult skulls, U. C. nos. 46466 and 42865. The juvenile skulls were prepared by the i-.se of ten per cent acetic acid and treatment with Glyptal as described by Brink (1957b). They are both crushed dorsoventrally, and lack the postorbital bars. Badly crushed and broken lower jaws are also associated with each of these skulls. U. C. no. 40466 is a fully adult skull; its preservation is perfect and undistorted, and very little is missing. It was manually prepared by Mr. Martin Caulkin, whose careful work on this specimen is gratefully acknowledged. The other adult specimen is somewhat smaller, and is also well preserved and nearly complete, but is slightly distorted. It was also prepared in acid as described above to expose the basicranium and the inside of the cranial cavity.
Brink (1955) has described an association of an adult and a juvenile skull of Thrinaxodon liorhinus found together in the same nodule. The juvenile skull is incomplete behind the orbits. Juveniles are uncommon in the fossil record and the completeness of the University of California specimens warrants a description supplementary to that of Brink.
The description is based on both skulls, and unless otherwise stated, the characters are preserved on both specimens.
The nasals resemble those of larger individuals in being narrow anteriorly and pitted with tiny foramina. Posteriorly they expand and articulate with the frontals, prefrontals, and lacrimals, though this region of the nasals is not as much expanded as in the adults.
The maxilla has the usual shape and contacts, and is also strongly pitted with foramina. The teeth will be discussed below.
The prefrontals are small and just touch the postorbitals. The latter are fragmentary, but indicate that the posterior extent of the postorbital was at the fronto-parietal suture or a little posterior to it. The postorbital bars are broken away. The jugals and squamosals closely resemble those of the adults.
The right quadrate is present on no. 42877, but it is badly crushed, and only a tiny dorsal spike of the quadratojugal remains in its groove in the squamosal.
The angulars are relatively a little larger and more flattened externally than in the adults. The angular flanges are broken away in both specimens.
Teeth in adult specimens are much larger than those of the juveniles. Replacement teeth in the juvenile are very little larger than those replaced, indicating that further replacements would be needed to bring the teeth up to the adult size. Brink (1955, pp. 75-76) came to the same conclusion on the basis of his small skull, and Crompton (1955, p. 665) has shown that some higher cynodonts also have multiple (i.e. non-mammalian) tooth replacement.
In contrast to adult cynodonts, the pterygoids enclose a small interpterygoid vacuity on each side of the cultriform process of the parasphenoid. Dorsally the basipterygoid processes are smooth, and articulate on correspondingly smooth vertical faces of the pterygoids, just forward of the internal, carotid foramina ventrally, the median ridges of the pterygoids curve mediad, do not meet the cultriform process, and have a digitate suture with the basipterygoid processes. The ventral keels of the anterior portions of the pterygoids are not continued posteriorly onto the rounded basipterygoid tubera. The pterygoids continue Posteriorly, but are broken before reaching the quadrates.
The basisphenoid and parasphenoid are fused, except at the anterior end of the basisphenoid dorsally, where a slight separation is present in the region of the trabecular attachment to the basisphenoid, as described by Parrington (1935b, p. 400). The tip of the cultriform process of the parasphenoid is sutured anteriorly to the pterygoids, and the process extends posteriorly between the interpterygoid vacuities. At this point the cultriform process bears a ventral keel, and in the region between the prominent internal carotid foramina, it expands and bears five small teeth on a roughened area. From this area, the wings of the parasphenoid expand and pass back over the basioccipital in a squamous suture, the full extent of which is obscured by breakage. There is a small gap between the basioccipital and basisphenoid, which was undoubtedly filled with cartilage.
The basioccipital is a hexagonal bone, and bears paired oval depressions on the ventral surface, which were interpreted by Parrington (1946b, p. 186) as attachments for the rectus capitis anticus muscles. These depressions are relatively much deeper than those of larger specimens. In no. 42877, the left one has a foramen opening into its lateral wall, facing medially and a little posteriorly. This foramen opens into a canal, which disap-
pears into the unossified area between the prootic and basioccipital. The foramen and its canal are formed wholly within the basioccipital. This is certainly the same structure as that described by Watson (1913, p. 220) in Diademodon, and is, as he suggests, probably venous. On the posterior slope of each muscle pit is a smaller foramen which is directed towards the occipital condyles. These are also visible in the adult (see PI. 1, fig. 2). In addition, a tiny foramen is present on the opisthotic about midway between the posterior borders of the jugular foramen and the fenestra ovalis.
juvenile. Figure 3B shows that in the juvenile, a process of the opisthotic almost completely closes the fenestra ovalis anteriorly; thus about three- fourths of the fenestral border is formed by the opisthotic, the remainder by prootic. A parasphenoid contribution to the fenestral border is small, if present, and the fenestra does not reach the basioccipital.
Posteriorly, the fenestra ovalis is confluent with the jugular foramen through a distinct channel, which lies entirely within the opisthotic and emerges within the jugular foramen near its external opening. It was apparently a complete canal in life, and must represent the fenestra rotunda. The presence of a fenestra rotunda was first demonstrated in therapsids by Simpson (1933, p. 289) in Nythosaurus. Olson (1944, p. 25) suggested that the perilymphatic duct in therapsids opens into the jugular foramen. These juvenile specimens demonstrate that this was the case in Thrinaxodon liorhinus, and that a fenestra rotunda was present in cynodonts more primitive than Nythosaurus.
Anterior to the fenestra ovalis, a deep recess is present in the skull base. It is formed principally in the basioccipital, except for its anterolateral and posterolateral corners, which consist of prootic and opisthotic, respectively. Apparently a thin cartilage coating was present on the interior of this recess, but its definite shape and relatively smooth inner surface indicate that it housed a soft structure and was not cartilage filled. The recesses were probably covered by parasphenoid in life, as in the adult, but breakage has now exposed them on no. 42877. The recess is partially roofed laterally by a small process of the prootic, which tends to separate it from the saccular recess (see below, and Fig. 3B). The recess in the basioccipital is present only as a slight concavity in the adult. In no. 42865 (a young adult in which the braincase was prepared by acid) the area is very slightly concave, but no distinct recess is present. The conformation of the fenestra ovalis is very similar, in the young adult, to that described by Parrington (1946). However, in no. 42865, the anterolateral notch, in the large opening described by Parrington as the fenestra ovalis, has a smooth, finished edge internally and is confluent with the area immediately ventral to the anterior ampulla. This particular notch is probably the ventral edge of the saccular recess, while the recess in the basioccipital is certainly a receptacle for a cochlear apparatus. Relative reduction of the basioccipital recess in the adult is probably a result of early development of the ear region of vertebrates and very little increase in size as adult size is reached.
The jugular foramina are large, formed between the exoccipital and opisthotic, and on their concave posteromedial borders a pair of small foramina are present. These are confluent with the condylar canal, and carried the hypoglossal nerves.
The stapes is roughened and unfinished proximally, and evidently bore a heavy cartilage plug, fitting into the slightly bevelled sides of the fenestra ovalis. It was perhaps similar to the ossified plug described by Parrington (1955, p. 14) in Scylacops capensis. Only about one-half of the articular end of the stapes Covers the fenestra ovalis. The remainder projects into, and partly over, the opening into the so-called "unossified region" Parrington, 1946b, p. 185) and thus comes near the lateral wall of the cochlear recess. The distal end of the stapes is unossified. There is a relatively large stapedial foramen, and the posterior limb of the stapes is slenderer than the anterior limb, the latter condition resembling that of the adult. The fenestra ovalis is separated from the "unossified region" by an anterior process of the opisthotic. Anteriorly, the vestibule of the fenestra ovalis is confluent with the cochlear and saccular recesses.
The ossification is light, more so than might be expected in the adult of a smaller species. The bone is very cancellous and translucent.
All sutures are clearly visible. This is also often true of the adults, but the latter have well-knit, occasionally complex and inter- digitated sutures, while those of the small specimens are relatively more open and have less complication.
The presence of small interpterygoid vacuities can be explained by juvenility of the specimens. Presumably the pterygoid musculature was not yet well developed; in the adult, these muscles obliterate the vacuities by appression of the medial flanges of the pterygoids against the midline. In the adult specimen discussed below (see also Pl. 1, fig. 1), the former position of the interpterygoid vacuities shows as two slim grooves on each side of the midline.
The presence of parasphenoidal teeth in a therapsid is unexpected. Palatal teeth on pterygoids and palatines are found in gorgonopsians as well as in other groups of mammal-like reptiles, and Vaughn (1958) has described sub-sphenoidal teeth in a small pelycosaur. These teeth may be more consistently present in therapsids than previously supposed. Perhaps they have passed unnoticed owing to removal by mechanical preparation or obscured in the older individuals by appression of the pterygoids to the midline.
Differences in tooth number have been considered to be taxonomically significant, but individual variation between right and left sides in both juveniles and adults vitiates its utility. An example of ontogenetic variation is provided by the comparison of the greater number of juvenile than adult teeth in Thrinaxodon liorhinus with the converse situation in Galesaurus planiceps (Rigney, 1938, p. 512).
In animals which, as adults, possess well- developed sagittal crests, the lack of development of these crests in juveniles is a frequent phenomenon, both in reptiles and mammals. Thus, the weak temporal crests, relatively large parietal foramen, and flattened skull table between the temporal crests of these small specimens indicate their juvenility.
The principal difference between the University of California specimens and Brink's juvenile one (Brink 1955, p. 73) is in degree of development of the anterior projections of the frontals. These spines, which thrust a wedge anteriorly between the nasals, are also found in Glochinodontoides, Platycranicllus, and Galesaurus. This is apparently a variable character in the juveniles of Thrinaxodon liorhinus, disappearing in the adults owing to further growth of nasals and frontals.
The other differences between adult and juvenile, pointed out by Brink, are present in the University of California specimens, though the presence of incomplete postorbital bars cannot be determined in this material. The relatively very large frontal of the juvenile is separated from the orbital margin by a very narrow meeting of the postorbital and postfrontal. The posterior margin of the secondary palate is at the level of the fifth tooth in the juvenile rather than the third as in the adult. The latter may be explained by the greater relative growth of the snout in the adult, and the greater number of anterior teeth in the juveniles.
Van Valen (1960. p. 306) mentions this vascularization of the snout region in Tupinambis, but suggests that the foramina "from their positions and relative development to be mostly related to the development of the teeth." On the contrary, these external maxillary (superior labial and lateral ethmoidal) foramina of lizards transmit only nerves and blood vessels serving, cutaneous structures. Dorsally, the foramina perforating the nasals and dorsal part of the nasal processes of the maxillae transmit cutaneous branches of the lateral ethmoidal nerve. These serve highly vascularized and richly innervated skin thickenings which surround the cartilaginous nasal tube (Oelrich, 1956, p. 88). All of the snout skin is firmly attached to the underlying bone, with the exception of the above-mentioned thickened area, which is slightly motile (ibid., p. 87), though no more so than in any other lizard.
The labial foramina transmit cutaneous branches of the superior alveolar nerve and maxillary artery, both serving the skin of the lower snout and the lip (ibid., pp. 62-63). The latter is very weakly developed in all lizards. Those branches of the maxillary artery and superior alveolar nerve which serve the teeth are transmitted through ventrally and internally opening foramina in the palatal shelf of the maxilla, and are completely separate from branches serving cutaneous structures.
The similarity of bone vascularization in Thrinaxodon and Tupinambis suggests, then, that these foramina in themselves may not be sufficient evidence to indicate the presence of associated extensive secretory, sensory, or muscular structures in Thrinaxodon or indeed in any other theriodont. Presence of numerous large foramina on the lower part of the maxilla near the alveolar border would, on the other hand, seem to preclude the presence of an extensive movable muscular cheek and lip. The vascular and nervous structures associated with these foramina would probably serve skin fairly closely united with the bone, for extensive movement of this skin would be disadvantageous. A muscular cheek, then, is probably to be correlated with the development of a posteriorly placed, single, external, infraorbital foramen as seen in mammals. So far as I am aware, no such structure, or even a tendency for such a grouping or codification of nervous and vascular snout structures is present in any theriodont.
In summary, this discussion does not suggest that rhinarium, hair, muscular cheeks and mobile lips were not present at all in some therapsids; rather, that at least some of the evidence which has been adduced for the presence of these or similar structures is necessarily inconclusive. An excellent summary of this evidence is given in Van Valen (1960).
Brink, Adrian S. 1955. Note on a very tiny specimen of Thrinaxodon liorhinus. Palaeontol. Africana, vol. 3, pp. 73-76, 1 fig.
----- 1957a. Speculations on some advanced mammalian characters in the higher mammal- like reptiles. Palaeontol. Africana, vol. 4, pp. 79-96, 5 figs.
----- 1957b. On the uses of Glyptal in paleontology. Palaeontol. Africana, vol. 4, pp. 124-130.
Crompton, A. W. 1955. On some Triassic cynodonts from Tanganyika. Proc. Zool. London, vol. 125, pp. 617-669, 15 figs.
Oelrich, Thomas M. 1956. The anatomy of the head of Ctenosaura pectinata (Iguanidae) Univ. Mich. Misc. Publ. Mus. Zool., no. 94, pp. 1- 122, 59 figs.
Olson, Everett C. 1944. Origin of mammals based upon cranial morphology of the therapsid suborders. Spec. Pap. Geol. Soc. Amer., no. 55, ix +136 pp., 27 figs.
Parrington, F. R. 1935. A note on the parasphenoid of the cynodont Thrinaxodon liorhinus Seeley. Ann. Mag. Nat. Hist., ser. 10, vol. 16, pp. 399-401, 1 fig.
-----1946. On the cranial anatomy of cynodonts. Proc. Zool. Soc. London, vol. 116, pp. 181-197, 10 figs.
----- 1955. On the cranial anatomy of some gorgonopsids and the synapsid middle ear. Proc. Zool. Soc. London, vol. 125, pp. 1- 40, 15 figs.
Rigney, Harold W. 1938. The morphology of the skull of a young Galesaurus planiceps and related forms. Jour. Morphol., vol. 63, pp. 491-529, 8 figs., 6 pls.
Simpson, George Gaylord 1933. The ear region and the foramina of the cynodont skull. Amer. Jour. Sci., ser. 5, vol. 26, pp. 285-294, 5 figs.
-----1938. Osteography of the ear region in monotremes. Amer. Mus. Novit., no. 978, pp. 1-15, 7 figs.
Van Valen, Leigh 1960. Therapsids as mammals. Evolution, vol. 14, no. 3, pp. 304-313.