


Researching whelks in Japan: Field notes from Jann Vendetti
By UCMP grad student Jann Vendetti, July 21, 2009: A research summary
| Sponsored by the NSF (National Science Foundation) and the JSPS (Japanese Society for the Promotion of Science), Jann is studying fossil and living whelks — a type of snail — in Japan, the home of more than one third of all extant whelks. Jann is interested in the evolution and dispersal of whelks through time and in their different modes of developmental growth. For more photos and more details of Jann's adventures in Japan, visit her blog. |
July 21, 2009: A research summary
I returned to Berkeley on August 21, 2008 — 11 months ago today — and I'm now in the final months of writing my dissertation. To see lots more photos from Japan and to read about my later adventures there, please see my blog, but here I'd like to provide a review of my Japan field work and share some of the results that came out of that trip.
There were two parts to my work in Japan:
Visiting museum collections to take photos and make molds of protoconchs (at the very top of a snail's shell) from snails in the family Buccinidae. The photos were for confirming identifications of the snails that I found in fish markets and in other museums, and the protoconchs were for helping answer development questions about the Buccinidae.
Visiting fish markets around the country to obtain live specimens for tissue sampling. With the tissue samples I could do DNA analysis and work out a buccinid phylogeny hypothesis.
Each of these parts evolved into two dissertation chapters: one on making the protoconchs with the molding and casting method and the other on molecular biology.
Protoconch molding
I visited a number of museum collections and shell museums where I made protoconch molds (using a common dental molding material) for more than 200 fossil and extant shells. I mentioned in my introduction that whelks exhibit two primary modes of development: some have a planktonic larval stage while others have larvae that emerge from their egg capsules looking like miniature versions of the adult snails. I am hoping to differentiate between these two modes of development by looking at the protoconch — a small protoconch presumably indicates planktonic development. With my broad sampling of protoconchs, I can test this presumption and hopefully answer the question "How did these species of snails develop?" I am still working with the numbers, but if I do find a correlation between protoconch size and developmental strategy, then I can use those data to infer development in fossil forms! It's unusual to be able to answer developmental biology questions about extinct organisms, but we can do it with snails, thanks to their great fossil record. I could also ask how many extinct forms develop in a certain way versus how many extant species develop that way now; what sorts of lineages started off with a planktonic developmental type but are now non-planktonic; and the big question: how does development evolve? — these are exciting questions yet to be explored. And it's something that my dissertation project has primed me for.
 |
 |
 |
||
Specimen photos
In Japan I took my own photos of all the specimens I looked at because the guides to Japanese buccinids didn't necessarily have them — I have so many pictures now that I could make my own guide! Once back in California, I printed out my pictures — a big stack of something like 300 photos — and using eight or so reference guides, I checked to make sure all the identifications were correct … and found names for a number of specimens that lacked them (not all museums had names for the specimens in their collections). I would say "So this is supposed to be this species …" and then check the guides to see what they said. If the identification appeared to be correct, then I would note it in my spreadsheets and keep track of the IDs that way. But there are still some that I could not identify in the buccinid literature — perhaps they are new species.
Collecting specimens for tissue sampling
As I've noted earlier, more than one third of all living whelks are found in the waters surrounding Japan. This fact alone would be reason enough for my going there to collect tissue samples, but then there is the little problem of specimen collection. A lot of whelk taxa are subtidal and can live down to a thousand meters — even if I were a licensed SCUBA diver, many would be out of my reach. And diving for specimens in another country, especially with my limited knowledge of the language, would have added another layer of difficulty. But fortunately for me, the Japanese like to eat whelks — they trawl for or trap them and then sell the live snails in the plentiful fish markets across the country — so it was these two things: Japan's rich whelk fauna and the ease with which I could obtain samples that led me to Japan.
But obtaining specimens in fish markets presented two more problems: identification and preservation. Identification because it was important to know what it was I was buying. Without a clear search image of the different genera and species, I could easily waste a lot of money buying up every available snail. Sometimes the shells just look like … "shells" … if you haven't been staring at them long enough. So I did a lot of preparatory homework, studying all the Japanese reference guides I could find and learning to differentiate the animals by shell characteristics.
Then there was the preservation problem: how do I preserve my specimens until I can deal with them back at the Nagoya University lab? It wouldn't be a big deal if I could take my small tissue samples during my travels and discard the shells, but I had to keep the shells too. I would be determining species based on shell characteristics so I had to keep the shells as voucher specimens — if somebody were to question my identifications, I could produce the shells to show where I got the tissue samples.
At first I thought that I'd have to do day trips only — buy the snails at the market and come right back to do the lab work: freeze them, take tissue samples, put them in alcohol to preserve the DNA, then remove the animals and clean out the shells. But as it turned out, preserving the specimens was a non-issue. Japan has a very inexpensive and convenient frozen transport system available at all the fish markets — because people have their fish market purchases frozen and then shipped to their homes (or wherever) all the time. So I had my live specimens packed in a freezer truck and delivered to the university — and when I got back to Nagoya, they were there waiting for me in the lab freezer.
Molecular biology — working out a buccinid phylogeny
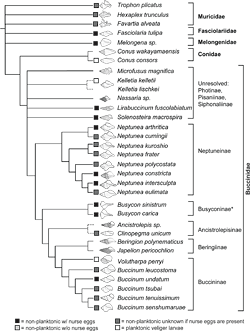 My hypothesis of phylogenetic relationships of selected buccinids based on the molecular data I obtained from tissue samples. Click on the image to see an enlarged version. |
So I took tinier samples from my tiny samples, amplified the DNA, and then sequenced it for two different genes: a slow-evolving gene and a fast-evolving gene. The purpose of this was to try and get the "inner branches" of the family tree (or phylogeny) resolved, and then with the fast gene, to resolve the "tips" of it. Suppose two genera are closely related — the slow-evolving gene would tell you that, but it might not tell you how closely related the species are, because if it evolves slowly, maybe two of those will have the same sequence. The slow gene and the fast gene in concert are different kinds of data that complement each other, so when you create a phylogeny from those gene sequences, you can be confident that your phylogenetic hypothesis is reflecting a more complete picture of the relationships between those groups than from one gene alone.
We can't tell whether a species has a planktonic or non-planktonic developmental strategy by looking directly at the DNA (not yet anyway) but it can tell us something about development indirectly. Once you work out the phylogeny from your DNA analysis, you can then map on characteristics — like those developmental strategies. Where those fall on the tree is what really interests me. I've mapped them onto my phylogeny and different development types show up throughout the tree — evidence that developmental type is an evolvable trait.
With a bigger sample size I'd be able to say a lot more about that and be able to reconstruct ancestral character states using Bayesian analysis, a technique developed by John Huelsenbeck (in our Department of Integrative Biology). This would be a fascinating project to do!
The importance of field work
I'd like to make a final comment about the value of field work in scientific research. For a lot of biologists and paleontologists field work is the foundation of your science — the most important part of your whole project. The "field" is primarily the place where you collect your data, but it's also where you think about your ideas, look at outcrops if you are a paleontologist or a geologist, or look at actual organisms in situ if you are a biologist. But the "field" can also be an artificial, human-constructed environment like a museum … or a fish market! For Jack Sepkoski, who put together huge compendia of first appearance/last appearance dates for genera and first appearance/last appearance dates for families for most of the Phanerozoic, the library was his "field" site.1 The actual "field" can be different for people seeking answers to different questions.
I still have lots of questions to answer about the Buccinidae — every answer I get seems to generate new questions. To conclude, I'd just like to say that there are still hundreds of buccinids out there that can't be found in fish markets — and virtually countless questions to be answered about the evolution of development and developmental evolution in fossil organisms.
1David Raup of the University of Chicago, in a tribute to Sepkoski, wrote "Jack often said that he was happiest with a pad of paper, computer, and a good library ('my field area')." From Raup, D.M. 1999. J. John Sepkoski Jr. (1948-1999). Paleobiology 25(3):424-429.
All photos by Jann Vendetti.