Written by Camilla Souto, August 2019; also see this July 2018 blog post
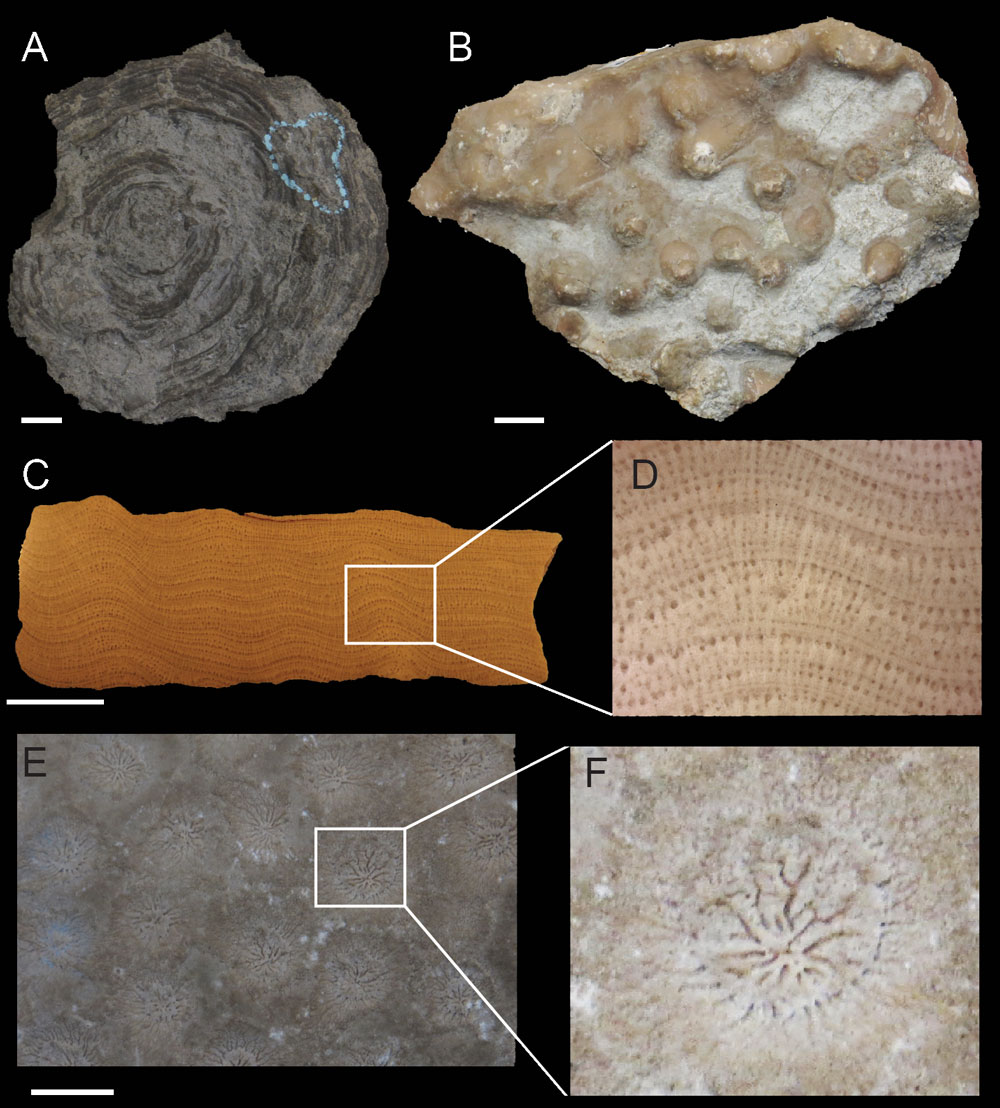
Stromatoporoids had a variety of morphologies (Figure 1A–B), such as dome-shaped, dendroid, finger-like, encrusting and tabular. Their size could vary from a few millimeters to massive reefs with domes up to five meters in diameter. They secreted calcium carbonate to form their skeleton in a reticulate structure; however, it’s unknown whether the original composition was calcite or aragonite. The skeleton was made of horizontal layers (the laminae) supported by upright rod-like structures (the pillars) (Figure 1C–D). Between the laminae there were galleries filled in with sea water or soft tissue. The stromatoporoid grew upwards by adding sheets of laminae and the skeleton was quite variable across different taxa (Figure 2). Similar to other sponges, they also had internal canal systems (the astrorhizal canals) that communicated with the external environment for filter-feeding. The canal system can be seen on the outside of the stromatoporoid body as a series of elevations (the mamelons; Figure 1B) with striation marks (the astrorhizae; Figure 1E–F).
Apparently, the growth rate and resulting shape of the stromatoporoids were plastic and influenced by changes in the environment. For instance, a computer simulation of their skeletal growth suggested that the patterns of sedimentation played an important role on the stromatoporoid growth and that a pause in sedimentation was needed for their successful establishment. Other environmental factors suggested to influence their plasticity are tidal or seasonal fluctuations, paleocurrents, changes in temperature or nutrient regimes, water turbidity, and consistence of the sea floor.
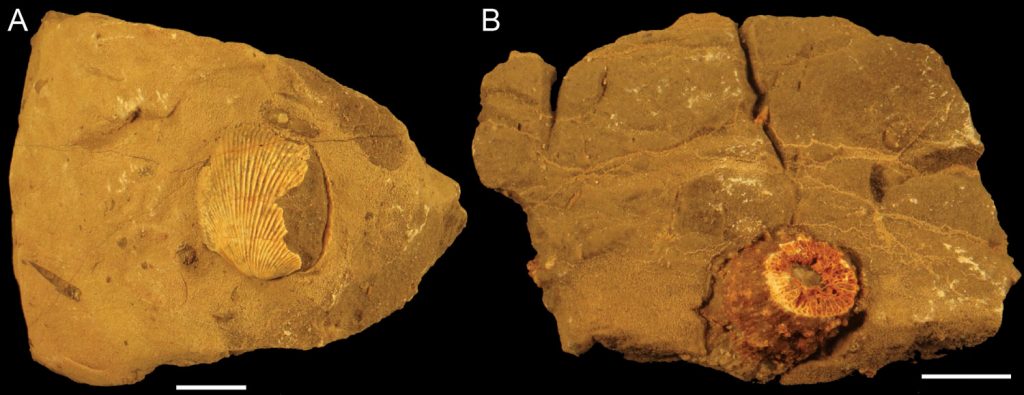

Rugose corals (Figure 4) have been found vertically oriented inside stromatoporoid skeletons (Figure 3B), which suggest they often lived in a symbiotic relationship. The surface of the stromatoporoid would provide a stable growth substrate for the rugosan and also an elevated position from the sea floor that would probably enhance feeding (rugose corals were carnivores). This association is usually referred as commensal but its effect on the stromatoporoids is not known. Therefore, parasitism and mutualism have not been ruled out. For instance, it has been suggested that the feeding efficiency of the stromatoporoid was reduced because the rugosan would obstruct the astrorhizal canals, thereby reducing the water flow. But it is also possible that the rugosans kept small predators away from the stromatoporoid. Some rugosans have also been found completely embedded in the stromatoporoid skeleton.
All of the reef builders mentioned above were extinct in the Paleozoic, and they were mainly substituted by the scleractinian corals that originated in the Triassic. These corals are the main reef builders today and it has been that suggested that their success is a result of their association with the zooxanthellae since the mid-Jurassic. Zooxanthellae obtain energy through photosynthesis and their association provided an input of nutrients to the scleractinian coral, resulting in increased growth rates and better integration of the corallites. However, oxygen isotopic data suggest that tabulate corals also had such associations since the Middle Silurian and associations with photosynthetic organisms could have been present in other groups. For instance, some groups of stromatoporoids probably lived in association with cyanobacteria.
Stromatoporoid and scleractinians had contrasting growth strategies to cope with hydrodynamic forces at the turbulent reef zone. The former had slow growth rates and formed a resistant skeleton, while the latter are more fragile and compensate their breakage by rapid growth promoted by its association with the zooxanthellae. This strategy probably allowed scleractinians to colonize more places; stromatoporoids, on the other hand, were limited to environments with particular characteristics that maximized their growth.
Diversity dynamic studies suggest that the origination rate of the stromatoporoids in the Early–Middle Devonian exceeded the extinction rate, promoting their burst of diversification; however, this pattern was flipped afterwards. Negative net diversification then reduced the stromatoporoid diversity over time culminating on the extinction of this group. A possible reason for this decline is the rise of sea level. Stromatoporoids are mainly from shallow water and a higher sea level would take them away from their optimal water depth. Slow growth rates would not be able to cope with this change and many groups would have gone extinct as a result. The remaining groups were then affected by the global cooling in the Late Denovian resulting in the extinction of the whole group in the Early Carboniferous. Other studies also suggest that anoxic conditions were important for their deminse and also biotic causes related to bacteria infestation. However, much work needs to be done to evaluate the possible causes of their demise.
Information about stromatoporoids in the literature is very controversial, especially because their morphology is quite different from modern sponges. For instance, because they are heavily calcified and formed reefs, they were first thought to be hydrozoans (Cnidaria). Also, some stromatoporoid species were first described as red algae! The first comparisons of stromatoporoid specimens to sponges were made by Baron von Rosen in 1869, and then Nicholson & Murie in 1878, based on morphological similarities with the sclerosponges (Demospongiae sponges; similarities were mainly the gross skeletal pattern and the presence of a canal system for water filtration). However, no spicules were found on the stromatoporoids and this idea was not easily accepted. The stromatoporoids became part of the phylum Porifera only 100 years later, when calcareous sponges were discovered and spicules were found in some specimens. The reclassification was formalized by Hartman and Goreau in 1970; since then, suggestions for dropping the cnidarian nomenclature used to describe stromatoporoids were made. Unfortunately, some textbooks still use inappropriate terms such as “coenosteum”, a word that refers to the skeleton secreted by cnidarians. Currently, it is widely accepted that stromatoporoids are sponges and some living calcified sponge genera such as Astrosclera and Stromatospongia actually have skeletons very similar to the stromatoporoids. Their classification within the phylum Porifera has also been controversial. Some researchers classify the Paleozoic stromatoporoids without spicules on their own class Stromatoporoidea, while others classify all of them as coralline Demospongiae because of the presence of siliceous spicules in some groups.
In addition, the term stromatoporoidea sensu lato does not include a monophyletic group (i.e., they do not share a common ancestor). Mesozoic groups that have been referred to the stromatoporoids are polyphyletic and most are possibly different lineages within the Demospongiae. Although they have some similar characteristics with the stromatoporoids, they usually have vertical skeletal structures, instead of horizontal like the Paleozoic stromatoporoids. As a result, their stratigraphic range in the literature is very variable. Stromatoporoid sensu stricto include only the Paleozoic groups, and their monophyly has not been tested yet.
Taxonomic studies on the stromatoporoids were abundant in the 19th century. The high interest on this group was probably a result of its importance for the oil and gas industry. The petroleum extracted from Silurian and Devonian strata are mainly from stromatoporoid origin!! Many studies were also done during the 1950–60s when Russell Waines, a UC Berkeley graduate student, wrote his dissertation about the stromatoporoids of the Late Devonian of Nevada (and following the Treatise of Invertebrate Paleontology, he classified them as cnidarians). Nevada apparently hosted a great diversity of stromatoporoids during the Devonian, and they were first recognized by Charles Doolittle Walcott, the same paleontologist that discovered the Burgess Shale fossils in the Cambrian rocks of British Columbia, Canada!!
References
Boardman, R.S.; Cheetham, A.H.; Rowell, A.J. 1987. Fossil Invertebrates. Blackwell Scientific Publications. 713 pp.
Stock, C.W. 2001. Stromatoporoidea, 1926–2000. Journal of Paleontology 75:1079–1089 (doi:10.1017/S0022336000017145).
Clarkson, E.N.K. 1998. Invertebrate Palaeontology and Evolution. 4th Edition. Blackwell Science. 452 pp.
Coates, A.G. & Jackson, J.B.C. 1987. Clonal growth, algal symbiosis, and reef formation in corals. Palaeobiology 13(4):363–378.
Deyuan, D. 1990. The rise, development and extinction of stromatoporoids. Palaeontologia Cathayana 5:267–268.
Hartman, W.D. & Goreau, T.E. 1970. Jamaican coralline sponges: their morphology, ecology, and fossil representatives. Zool. Soc. London Symposium. 25:205–243.
Kershaw S. 1987. Stromatoporoid–coral intergrowths in a Silurian biostrome. Lethaia 20:371–380.
Kershaw, S. 1998. The applications of stromatoporoid palaeobiology in palaeornvironmental analysis. Palaeontology 41(3):509–544.
Kiessling, W.; Aberhan, M.; Brenneis, B.; Wagner, P. 2007. Palaeogeography, Palaeoclimatology, Palaeoecology 244:201–222.
Łuczyński, P. 1998. Stromatoporoid morphology in the Devonian of the Holy Cross Mountains, Poland. Acta Palaeontologica Polonica 43(4):653–663.
Schuhmacher, H. & Plewka, M. 1981. Mechanical resistance of reefbuilders through time. Oecologia 49:279–282.
Stanley, G.D., Jr. 2001. The History and Sedimentology of Ancient Reef Systems. Topics in Geobiology Series Volume 17. Fluwer AcademicPlenun Publishers. 458 pp.
Stearn, C.W. 1972. The relationship of the stromatoporoids to sclerosponges. Lethaia 5:369–388.
Stearn, C.W.; Webby, B.D.; Nestor, H. & Stock, C.W. 1999. Revised classification and terminology of Paleozoic stromatoporoids. Acta Palaeontologica Polonica 44(1)1–70.
Stock, C.W. 2005. Devonian stromatoporoid originations, extinctions, and paleobiogeography: how they relate to the Frasnian-Famennian extinction. In: Over, D.J.; Morrow, J.R. & Wignall, P.B. Understanding Late Devonian and Permian-Triassic Biotic and Climatic Events: Towards an Integrated Approach. Elsevier. p. 71–92.
Swan, A.R.H. & Kershaw, S. 1994. A computer model for skeletal growth of stromatoporoids. Palaeontology 37(2):409–423.
Vinn, O. & Motus, M.A. 2014. Endosimbiotic rugosan symbionts in stromatoporoids from Sheinwoodian (Silurian) of Baltica. PLoS One 9(2):e90197 (doi:10.1371/journal.pone.0090197).
Vinn, O.; Wilson, M.A.; Toom, U.; Motus, M-A. 2015. Earliest known rugosan-stromatoporoid symbiosis from the Llandovery of Estonia (Baltica). Palaeogeography, Palaeoclimatology, Palaeoecology 431:1–5.
Wendt, J. & Kaufmann, B. 2006. Middle Devonian (Givetian) coral-stromatoporoid reefs in West Sahara (Morocco). Journal of African Earth Sciences 44:339–350.
Wilson, E.C.; Waines, R.H. & Coogan, A.H. 1963. A new species of Komia Korde and the systematic position of the genus. Palaeontology 6(2):246–253.
Wood, R.A. & Reitner, J. 1986. Poriferan affinities of Mesozoic stromatoporoids. Palaeontology 29(3):469–473.
Wood, R. 1987. Biology and revised systematics of some late Mesozoic stromatoporoids. Special Papers in Palaeontology 37:1–89.
Wood, R. 1990. Reef-building sponges. American Scientist 78(3):224–235.
Wood, R. 1998. The ecological evolution of reefs. Annual Reviews in Ecology and Evolution 29:179–206.
Yiming, G.; Ran, X.; Zhongdao, T.; Yuanlan, S. & Baohua, L. 2005. Relationships between bacterial-algal proliferating and mass extinction in the Late Devonian Frasnian-Famennian transition: Enlightening from carbon isotopes and molecular fossils. Science in China ser. D Earth Sciences 48(10):1656–1665.
Zapalski, M.K. 2014. Evidence of photosymbiosis in Palaeozoic tabulate corals. Proceedings of the Royal Society B 281: 20132663 (doi: 10.1098/rspb.2013.2663).