|
||||
Creating an Earth System II:
Learning about the Ozone |
||||
Reviewing the Earth's Atmosphere - The Radiation MediumOrigin of the Atmosphere
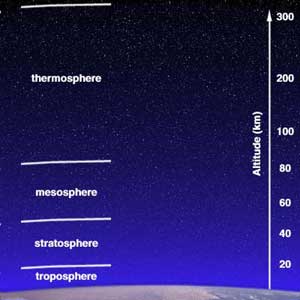
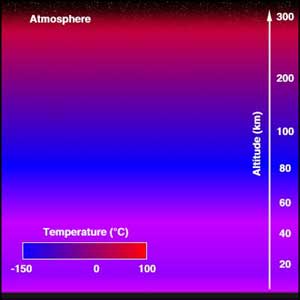
Structure of the AtmosphereThe atmosphere consists of subdivisions according to temperature:
The Ozone LayerThe Sun's UV radiation is absorbed by the ozone layer. Ozone consists of oxygen atoms bonded in threes (O3); we breathe oxygen that's bonded in pairs, or O2. Ozone forms in the mid-stratosphere. Without the ozone layer, the Earth's surface would be sterilized by UV radiation. The breakdown of the ozone layer increases skin cancer and cataracts in humans, impairs immune systems of all animals (including humans), and interferes with phytoplankton productivity in the oceans. Humans have impacted the ozone layer: Chlorofluorocarbons (CFCs) used for refrigeration, cleaning solvents, and aerosol sprays since the 1950s help destroy ozone. Ozone became strongly depleted over the Earth's polar regions in the later part of the 20th century. Video: OzoneThis video Resource: Sun Splash Ozone This NASA video explains ozone depletion using computer graphics and animation. An educational narrative explains how stratospheric ozone protects us from UV radiation and demonstrates how chlorofluorocarbons cause destruction of the Earth's ozone layer. The video is appropriate for grades 5-8, 7 minutes in length, closed-captioned, and sells for $10.00. It is available from NASA CORE: Visit NASA CORE or e-mail: nasaco@leeca.org. Activities:
|
||||
|
Return to: Creating an Earth System II | Dynamic Earth Homepage | UCMP Homepege |